Business
NAFDAC DG PAYS A WORKING VISIT TO EMZOR CAMPUS

NAFDAC DG PAYS A WORKING VISIT TO EMZOR CAMPUS
About Emzor
Sahara Weekly Reports Emzor is a privately owned indigenous pharmaceutical company founded in 1984 by Dr. Stella Okoli. The Company has grown into a legacy pharmaceutical company with 4 modern factories that manufacture over 140 world-class pharmaceutical products, such as analgesics (the widely used Emzor Paracetamol), vitamins, haematinics, anti-malarial, anti-tussive, antibiotics, antihelminthic, anti-histamine, antacid, and cardio-protective drugs.

Emzor has a network of over 120 distributors covering public and private institutions across West Africa. Emzor is the largest indigenous pharmaceutical manufacturer in the country with over 120 products, all NAFDAC approved.
THE FACTORY VISIT
The NAFDAC Director General, Prof. Mojisola Adeyeye in company of Dr. Monica Eimunjeze (Director, Drug Registration And Regulatory Affairs (DRRA); Mrs Ijeoma Nwankwo, U. (Director, Drug Evaluation & Research (DER) and Dr. Gbenga Fajemirokun (SA-DG NAFDAC) visited the Ultra-Modern Pharma Factory known as Emzor Campus.
The World Health Organization (WHO) compliant factory is sited on more than 60 Hectares of land at the Shagamu Interchange of the Lagos – Ibadan Expressway and is the largest pharmaceutical facility in West Africa.
The factory is also (cGMP) compliant and has already manufactured and supplied millions of doses of medication ranging from antimalarials, paediatric care, vitamins and antiretrovirals to various international organizations through partnerships for public health intervention.
DURING THE VISIT
MRS. Uzoma Ezeoke the EDG. Emzor briefed the NAFDAC team on the Emzor Emzor group’s progress from inception to date. She said that the company has grown from the days of drug sales & marketing to manufacturing for the local and African market to exportation to UK and Netherlands in Europe and the United States of America.
This is in line with the Emzor group’s goal to be the number one name in pharmaceuticals on the African continent.
After the NAFDAC teams tour of the Factory; the team addressed their host, the Emzor group.
The NAFDAC DG, Professor Mojisola Adeyeye says “I taught pharmaceutical manufacturing in the United States for 19 years and I usually would take my students to pharmaceutical companies as an extension of the class because I believe in experiential. Part of the training is the facility tour.
This facility that we have seen today can stand beside any facility in the US. To see what Emzor is doing gladdens my heart and makes us know that we can do it”.
She commended the Emzor work force on their dedication and commitment to the vision of Dr. Stella Okoli for the pharmaceuticals industry in Nigeria as well as upholding the standards set by NAFDAC under the management’s new structure and standards in the last three years to regulate the pharmaceutical industry in line with global best practices regulatory bodies.
She particularly praised the Emzor groups consumer targeted production and Health, Safety and Environment (HSE) compliance. She said that Emzor is indeed a trail blazer of the industry and has indeed archived its vision of being the number one pharmaceuticals manufacturing company on the continent.
She concluded by affirming that Emzor has proven that anything is possible in Nigeria that whatever industry you operate in, if you build it to global standard the world will come to us.
Dr. Monica Eimunjeze (Director, Drug Registration and Regulatory Affairs (DRRA) also praised Emzor facility’s quality control and compliance with the NAFDAC regulations. She said that she had postponed her visit times before but is glad to have finally made it to the factory; that the world class facility is indeed a pride to the pharmaceutical industry and Nigeria.
She also praised the Emzor youth workforce that the future of the industry is assured with the training this young generation is being exposed at Emzor. That she’s particularly proud of the workforce especially the fact that the Emzor workforce is usually well represented like no other at NAFDAC organized trainings. She said that she expects an increase in the Emzor group international manufacturing partnerships.
Mrs. Ijeoma Nwankwo, U. (Director, Drug Evaluation & Research (DER) applauded the young workforce; the Vaccines and new Emzor API manufacturing Technology Transfer and licensing agreement with India’s Mangalam Drugs & Organic Limited to locally manufacture and distribute Active Pharmaceutical ingredients (4 API’s) for the treatment and prevention of malaria.
This leads to the development of a world class API manufacturing facility in compliance with international standards and the first of its kind in Sub- Saharan Africa.
Dr. Gbenga Fajemirokun (Special Assistant to the DG NAFDAC) also praised Dr. Stella Okoli for the young workforce and said seeing some of his old students working on such world class premises was a pride.
Dr. Stella Okoli thanked the NAFDAC team for taking the time out of their busy schedule to honor the Emzor family’s invitation to tour the WHO compliant facility. She reiterated that the Emzor group is committed to raising standards in the pharmaceutical industry in Nigeria and Africa.
Business
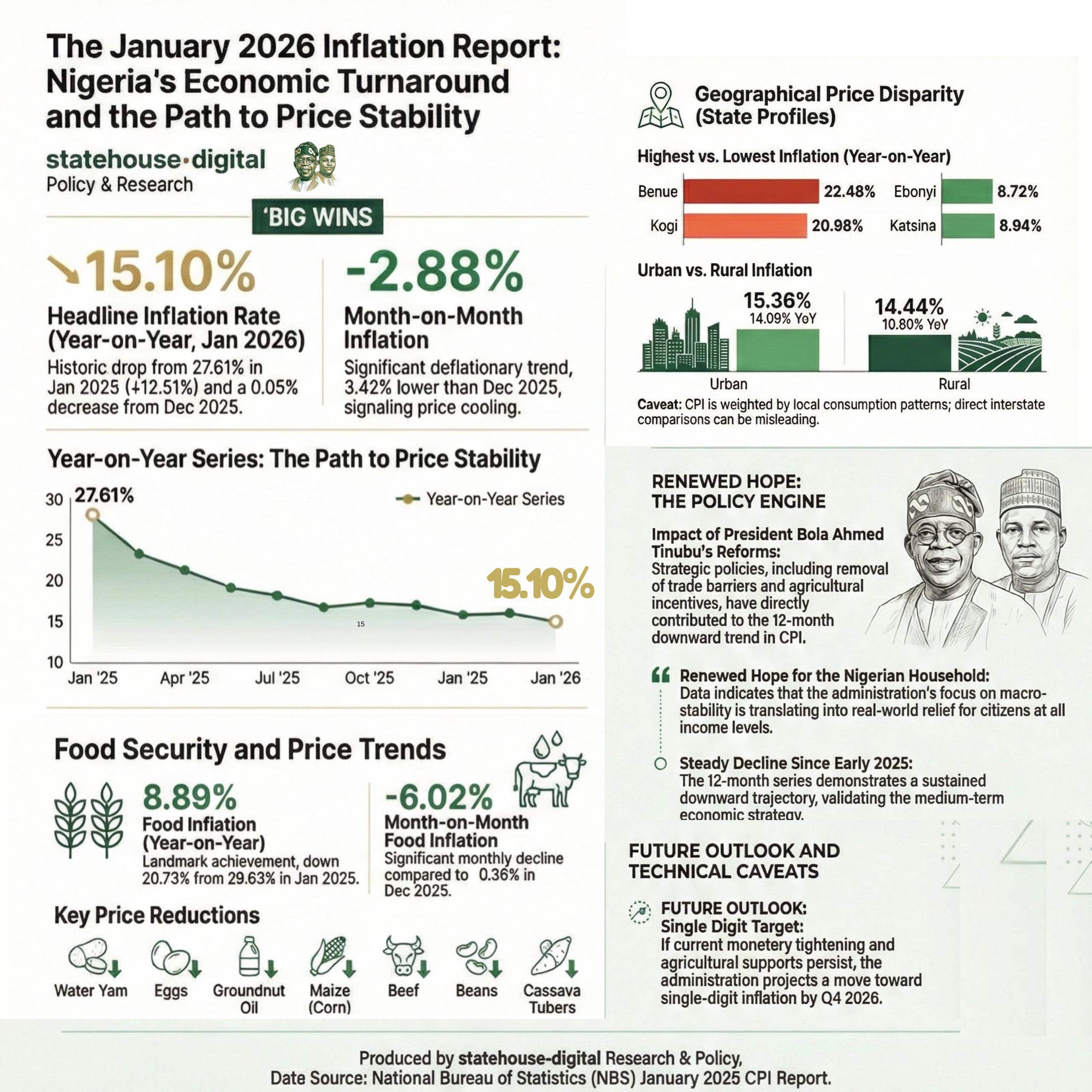
Nigeria’s Inflation Drops to 15.10% as NBS Reports Deflationary Trend

Nigeria’s headline inflation rate declined to 15.10 per cent in January 2026, marking a significant drop from 27.61 per cent recorded in January 2025, according to the latest Consumer Price Index (CPI) report released by the National Bureau of Statistics.
The report also showed that month-on-month inflation recorded a deflationary trend of –2.88 per cent, representing a 3.42 percentage-point decrease compared to December 2025. Analysts say the development signals easing price pressures across key sectors of the economy.
Food inflation stood at 8.89 per cent year-on-year, down from 29.63 per cent in January 2025. On a month-on-month basis, food prices declined by 6.02 per cent, reflecting lower costs in several staple commodities.
The data suggests a sustained downward trajectory in inflation over the past 12 months, pointing to improving macroeconomic stability.
The administration of President Bola Ahmed Tinubu has consistently attributed recent economic adjustments to ongoing fiscal and monetary reforms aimed at stabilising prices, boosting agricultural output, and strengthening domestic supply chains.
Economic analysts note that while the latest figures indicate progress, sustaining the downward trend will depend on continued policy discipline, exchange rate stability, and improvements in food production and distribution.
The January report provides one of the clearest indications yet that inflationary pressures, which surged in early 2025, may be moderating.
Bank
Alpha Morgan to Host 19th Economic Review Webinar

Alpha Morgan to Host 19th Economic Review Webinar
In an economy shaped by constant shifts, the edge often belongs to those with the right information.
On Wednesday, February 25, 2026, Alpha Morgan Bank will host the 19th edition of its Economic Review Webinar, a high-level thought leadership session designed to equip businesses, investors, and individuals with timely financial and economic insight.
The session, which will hold live on Zoom at 10:00am WAT and will feature economist Bismarck Rewane, who will examine the key signals influencing Nigeria’s economic direction in 2026, including policy trends, market movements, and global developments shaping the local landscape.
With a consistent track record of delivering clarity in uncertain times, the Alpha Morgan Economic Review continues to provide practical context for decision-making in a dynamic environment.
Registration for the 19th Alpha Morgan Economic Review is free and can be completed via https://bit.ly/registeramerseries19
It is a bi-monthly platform that is open to the public and is held virtually.
Visit www.alphamorganbank to know more.
Business
GTBank Launches Quick Airtime Loan at 2.95%

GTBank Launches Quick Airtime Loan at 2.95%
Guaranty Trust Bank Ltd (GTBank), the flagship banking franchise of GTCO Plc, Africa’s leading financial services group, today announced the launch of Quick Airtime Loan, an innovative digital solution that gives customers instant access to airtime when they run out of call credit and have limited funds in their bank accounts, ensuring customers can stay connected when it matters most.
In today’s always-on world, running out of airtime is more than a minor inconvenience. It can mean missed opportunities, disrupted plans, and lost connections, often at the very moment when funds are tight, and options are limited. Quick Airtime Loan was created to solve this problem, offering customers instant access to airtime on credit, directly from their bank. With Quick Airtime Loan, eligible GTBank customers can access from ₦100 and up to ₦10,000 by dialing *737*90#. Available across all major mobile networks in Nigeria, the service will soon expand to include data loans, further strengthening its proposition as a reliable on-demand platform.
For years, the airtime credit market has been dominated by Telcos, where charges for this service are at 15%. GTBank is now changing the narrative by offering a customer-centric, bank-led digital alternative priced at 2.95%. Built on transparency, convenience and affordability, Quick Airtime Loan has the potential to broaden access to airtime, deliver meaningful cost savings for millions of Nigerians, and redefine how financial services show up in everyday life, not just in banking moments.
Commenting on the product launch, Miriam Olusanya, Managing Director of Guaranty Trust Bank Ltd, said: “Quick Airtime Loan reflects GTBank’s continued focus on delivering digital solutions that are relevant, accessible, and built around real customer needs. The solution underscores the power of a connected financial ecosystem, combining GTBank’s digital reach and lending expertise with the capabilities of HabariPay to deliver a smooth, end-to-end experience. By leveraging unique strengths across the Group, we are able to accelerate innovation, strengthen execution, and deliver a more integrated customer experience across all our service channels.”
Importantly, Quick Airtime Loan highlights GTCO’s evolution as a fully diversified financial services group. Leveraging HabariPay’s Squad, the solution reinforces the Group’s ecosystem proposition by bringing together banking, payment technology, and digital channels to deliver intuitive, one-stop experiences for customers.
With this new product launch, Guaranty Trust Bank is extending its legacy of pioneering digital-first solutions that have redefined customer access to financial services across the industry, building on the proven strength of its widely adopted QuickCredit offering and the convenience of the Bank’s iconic *737# USSD Banking platform.
About Guaranty Trust Bank
Guaranty Trust Bank (GTBank) is the flagship banking franchise of GTCO Plc, a leading financial services group with a strong presence across Africa and the United Kingdom. The Bank is widely recognized for its leadership in digital banking, customer experience, and innovative financial solutions that deliver value to individuals, businesses, and communities.
About HabariPay
HabariPay is the payments fintech subsidiary of GTCO Plc, focused on enabling fast, secure, and accessible digital payments for individuals and businesses. By integrating payments and digital technology, HabariPay supports innovative services that make everyday financial interactions simpler and more seamless.
Enquiries:
GTCO
Group Corporate Communication
[email protected]
+234-1-2715227
www.gtcoplc.com
-

 celebrity radar - gossips6 months ago
celebrity radar - gossips6 months agoWhy Babangida’s Hilltop Home Became Nigeria’s Political “Mecca”
-

 society6 months ago
society6 months agoPower is a Loan, Not a Possession: The Sacred Duty of Planting People
-

 society5 months ago
society5 months agoReligion: Africa’s Oldest Weapon of Enslavement and the Forgotten Truth
-

 news6 months ago
news6 months agoTHE APPOINTMENT OF WASIU AYINDE BY THE FEDERAL GOVERNMENT AS AN AMBASSADOR SOUNDS EMBARRASSING