society
Pfizer, BioNTech Set To Eradicate Covid-19 With Vaccine
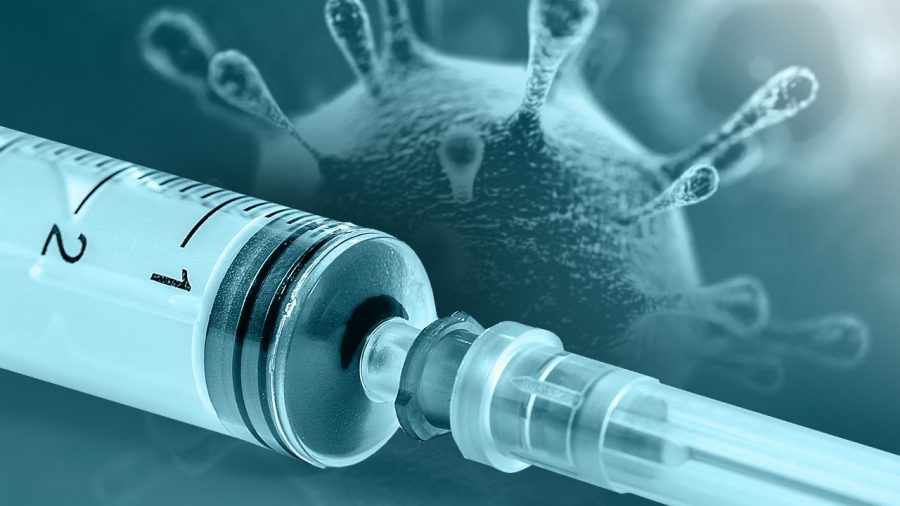
The battle for Victory over Covid-19 has received a major boost as Pfizer and partner BioNTech said Monday that their vaccine against Covid-19 was strongly effective, exceeding expectations with results that are likely to be met with cautious excitement — and relief — in the face of the global pandemic.

The vaccine is the first to be tested in the United States to generate late-stage data. The companies said an early analysis of the results showed that individuals who received two injections of the vaccine three weeks apart experienced more than 90% fewer cases of symptomatic Covid-19 than those who received a placebo. For months, researchers have cautioned that a vaccine that might only be 60% or 70% effective.
The Phase 3 study is ongoing and additional data could affect results.

In keeping with guidance from the Food and Drug Administration, the companies will not file for an emergency use authorization to distribute the vaccine until they reach another milestone: when half of the patients in their study have been observed for any safety issues for at least two months following their second dose. Pfizer expects to cross that threshold in the third week of November.
“I’ve been in vaccine development for 35 years,” William Gruber, Pfizer’s senior vice president of vaccine clinical research and development, told STAT. “I’ve seen some really good things. This is extraordinary.” He later added: “This really bodes well for us being able to get a handle on the epidemic and get us out of this situation.”
Although it is a bright spot in the battle against the pandemic and a triumph for Pfizer and BioNTech, a German company, key information about the vaccine is not yet available. There is no information yet on whether the vaccine prevents severe cases, the type that can cause hospitalization and death.
Nor is there any information yet on whether it prevents people from carrying the virus that causes Covid-19, SARS-CoV-2, without symptoms.
Without more information, it’s too early to start predicting how much of an impact the vaccine could make, said Michael Osterholm, director of the University of Minnesota’s Center for Infectious Diseases Research and Policy.
“I don’t want to dampen any enthusiasm for this vaccine. I just want us to be realistic,” Osterholm said. “For a vaccine to really have maximal impact, it’s going to have to also reduce severe illness and death. And we just don’t know yet.”
Because the vaccine has been studied for only a matter of months, it is impossible to say how long it will protect against infection with the virus. The vaccine does cause side effects, including aches and fevers, according to previously published data. Gruber said that he believed the side effect profile was comparable to standard adult vaccines, but probably worse than Pfizer’s pneumonia vaccine, Prevnar, or a flu shot.
The results have not been peer-reviewed by outside scientists or published in a medical journal, and even Pfizer and BioNTech have been given no other details about how the vaccine performed by the independent monitors overseeing the study.
Initial supplies of the vaccine, if authorized, will be limited. Pfizer says up to 50 million doses could be available globally. by the end of the year, with 1.3 billion available in 2021. There are also expected to be distribution challenges. The vaccine must be stored at super-cold temperatures, which could make it extremely difficult to deliver to many places. Pfizer has said it is confident those issues can be managed.
Although the estimate of the efficacy of the vaccine could change as the study is completed, it is close to a best-case scenario. That also bodes well for other vaccines in the late stages of testing, including those developed by Moderna, AstraZeneca, and Johnson & Johnson.
Both Pfizer’s vaccine and Moderna’s use messenger-RNA, or mRNA, technology, which uses genetic material to cause the body to create a protein from the virus; the immune system then recognizes the virus and learns to attack. Other vaccines in the late stages of development use genetically engineered viruses for a similar purpose, or pieces of protein that are directly injected. No mRNA product has ever been approved by regulators.
The story of how the data have been analyzed seems to include no small amount of drama. Pfizer, seeing an opportunity to both help battle a pandemic and demonstrate its research prowess, made decisions that were always likely to make its study the first of a Covid-19 vaccine to produce data — including its decision to have an independent group of researchers, known as a data safety and monitoring board, take an early look at the data in the 44,000-volunteer study before its completion.
The first analysis was to occur after 32 volunteers — both those who received the vaccine and those on placebo — had contracted Covid-19. If fewer than six volunteers in the group who received the vaccine had developed Covid-19, the companies would make an announcement that the vaccine appeared to be effective. The study would continue until at least 164 cases of Covid-19 — individuals with at least one symptom and a positive test result — had been reported.
That study design, as well as those of other drug makers, came under fire from experts who worried that, even if it was statistically valid, these interim analyses would not provide enough data when a vaccine could be given to billions of people.
In their announcement of the results, Pfizer and BioNTech revealed a surprise. The companies said they had decided not to conduct the 32-case analysis “after a discussion with the FDA.” Instead, they planned to conduct the analysis after 62 cases. But by the time the plan had been formalized, there had been 94 cases of Covid-19 in the study. It’s not known how many were in the vaccine arm, but it would have to be nine or fewer.
Gruber said that Pfizer and BioNTech had decided in late October that they wanted to drop the 32-case interim analysis. At that time, the companies decided to stop having their lab confirm cases of Covid-19 in the study, instead leaving samples in storage. The FDA was aware of this decision. Discussions between the agency and the companies concluded, and testing began this past Wednesday. When the samples were tested, there were 94 cases of Covid in the trial. The DSMB met on Sunday.
This means that the statistical strength of the result is likely far stronger than was initially expected. It also means that if Pfizer had held to the original plan, the data would likely have been available in October, as its CEO, Albert Bourla, had initially predicted.
Gruber said that there will not be another interim analysis conducted in the study. He also said that Pfizer’s estimate that it could file for authorization of the vaccine by the third week of November was based on the assumption that the FDA would be willing to accept two-month safety data on half the volunteers in the study as initially planned, when it was to include 30,000 volunteers, not more than 44,000, as is now the case. Those discussions are ongoing.
But Gruber said he now expects that by the time of the planned meeting of the FDA’s vaccine advisory committee in December, the study’s efficacy portion could be completed, having reached 164 cases of Covid-19.
He also emphasized that although there will only be a few months of data from this study, results from earlier studies make him optimistic that immunity from the vaccine will not wane rapidly.
The study has enrolled 43,538 volunteers, the companies said, and 38,955 have received their second dose. About 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds.
Bourla, Pfizer’s CEO, said the results mark “a great day for science and humanity,” in a statement, saying they provide “initial evidence of our vaccine’s ability to prevent Covid-19.” He added: “We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”
news
GEN CHRISTOPHER GWABIN MUSA SUPPORT INITIATIVE COMMENDS STATE-FEDERAL COLLABORATION IN ZAMFARA

GEN CHRISTOPHER GWABIN MUSA SUPPORT INITIATIVE COMMENDS STATE-FEDERAL COLLABORATION IN ZAMFARA
The Gen Christopher Gwabin Musa Support Initiative (GCGMSI) has commended the Zamfara State Government for its decisive contribution to security operations through the donation of newly acquired armoured personnel carriers (APCs), surveillance drones, and other critical operational equipment to troops and security agencies in the state.
This commendation was contained in a statement signed by the Convener of the GCGMSI, Ibrahim Dahiru Danfulani, Sadaukin Garkuwan Keffi/Betara Biu, and made available to the press.
The equipment was formally commissioned on Wednesday, February 18, by the Grand Patron of the GCGMSI and Minister of Defence, General Christopher Gwabin Musa, OFR (rtd.), in a ceremony at the Government House, Gusau. The event was attended by senior military officers, heads of security agencies, and top officials of the Zamfara State Government.
The GCGMSI, in its statement, hailed the donation as a “transformative and timely intervention” that aligns perfectly with its core objective of advocating for and supporting tangible measures that enhance the operational capacity and welfare of Nigeria’s security forces. The Initiative praised Governor Dauda Lawal’s administration for moving beyond rhetoric to actionable, material support, describing the move as a “blueprint for state-level collaboration in national security.”
“The provision of these assets by the Zamfara State Government is a testament to visionary leadership and a profound commitment to the peace and stability of its people,” the GCGMSI statement read. “It represents the exact kind of synergistic partnership between state and federal authorities that the GCGMSI champions. This initiative will significantly close operational gaps, boost the confidence of our gallant troops, and send a strong message to criminal elements.”
Speaking at the commissioning, General Musa emphasized that sustained collaboration is indispensable in confronting the nation’s evolving security challenges. He specifically commended Governor Lawal for his proactive support.
“Governor Dauda Lawal has demonstrated exemplary leadership and an unwavering dedication to the security of Zamfara State,” the Defence Minister stated. “The provision of these armoured vehicles, surveillance drones, and other operational equipment will undoubtedly boost the morale and operational effectiveness of our troops and other security agencies on the ground. This is a commendable effort that should be emulated by others.”
The newly commissioned assets, which include multiple APCs and advanced surveillance drones, are expected to dramatically enhance the mobility, protection, intelligence-gathering, and rapid response capabilities of security forces, particularly in the state’s remote and difficult terrains where anti-banditry operations are ongoing.
In his remarks, Governor Lawal reiterated his administration’s steadfast commitment to being a reliable partner in the security architecture. He urged security agencies to deploy the new resources responsibly and effectively to safeguard lives and property.
The Federal Government, through the Ministry of Defence, reaffirmed its commitment to continuing and deepening such partnerships with state governments across the nation to strengthen coordination and resource allocation in the collective fight against insecurity.
The GCGMSI concluded its statement by urging other state governments to take a cue from Zamfara’s “bold and pragmatic” approach, affirming that such concrete support is vital for achieving lasting peace and security across Nigeria.
society
Governor Dauda Lawal Commissions 25 Armoured Personnel Carriers, Aerial Surveillance Drones to Combat Insecurity

Governor Dauda Lawal Commissions 25 Armoured Personnel Carriers, Aerial Surveillance Drones to Combat Insecurity
In a major boost to the fight against banditry and insecurity in Zamfara State and the North-West Zone, Zamfara State Governor, His Excellency, Dr Dauda Lawal, on Wednesday commissioned 25 new Armoured Personnel Carriers (APCs) and sets of surveillance drones for the military and other security agencies operating in the state.
The event, which took place in Gusau, was part of the state government’s ongoing effort to provide structured logistical support to frontline security forces and combat insurgency, banditry, and protect lives and properties. Speaking at the commissioning and handover, Governor Lawal emphasised that the new assets are intended to enhance troop protection during high-risk deployments and improve rapid response capabilities in remote communities, ensuring tactical battle and overhead surveillance for victory.
“We have provided over 600 specialised motorcycles, 150 Hilux vehicles, and 20 Buffalo vehicles to our security forces. These 25 highly sophisticated APCs being commissioned today are therefore part of a broader reform to improve response to security threats. The APC’s significantly improves troop protection during deployments into high-risk areas. They reduce vulnerability during patrols, support convoy security along major routes, and strengthen rapid response capability when distress calls arise from remote communities.” the Governor stated.
Governor Lawal explained that the security challenges of recent years had disrupted farming, limited trade, and undermined public confidence across the state. He noted that his administration’s “Rescue Mission” agenda has focused on moving from fragmented responses to structured reforms, including the establishment of a Zamfara State Security Trust Fund and the operationalisation of Community Protection Guards to improve grassroots intelligence.
The Governor specifically highlighted the importance of integrating modern technology into security operations. He noted that the newly acquired drones would expand aerial surveillance, improve situational awareness, and support better coordination between command centres and troops in the field.
“Real-time information strengthens decision-making and reduces operational blind spots,” he added.
Governor Lawal however acknowledged the critical role of the Federal Government under President Bola Ahmed Tinubu, noting that recent federal budgets have allocated over three trillion naira to defence, a commitment he said strengthens subnational stabilisation efforts.
He urged the military commanders and personnel receiving the equipment to ensure disciplined maintenance and intelligence-guided deployment. “Enhancing your safety enhances the safety of our communities,” he told the troops.
Governor Lawal also told the people of Zamfara that; his administration remains resolute in restoring enduring security and peace across every Local Government Area. “We will sustain preventive measures, strengthen patrol architecture in rural corridors, deepen inter-state intelligence collaboration across the North-West, and maintain fiscal prudence in security expenditure. Stabilisation will continue through structured planning, lawful enforcement, and institutional reform.”
The Governor also linked the security investment to economic recovery, stressing that stability in rural areas is essential for agricultural productivity, market activity, and food security.
The event was attended by the Honourable Minister of Defence, General Christopher Musa (Rtd.), who formally commissioned the assets for operational service. Governor Lawal reaffirmed his administration’s resolve to sustain preventive measures and inter-state security collaboration until lasting peace is restored across all Local Government Areas in Zamfara.
society
Ramadan, Lent: Ajadi Urges Religious Harmony, Prayers for Nigeria

Ramadan, Lent: Ajadi Urges Religious Harmony, Prayers for Nigeria
A leading governorship aspirant of the Peoples Democratic Party (PDP) in Oyo State, Ambassador Olufemi Ajadi Oguntoyinbo, has extended warm felicitations to Muslims and Christians on the simultaneous commencement of Ramadan and Lent.
Ramadan, the Islamic holy month marked by 30 days of fasting and spiritual devotion, and Lent, the 40-day Christian season of fasting and reflection, began on the same day — a development Ajadi described as symbolic and spiritually significant.
In a statement personally signed by him on Wednesday, Ajadi congratulated adherents of both faiths and called for sustained religious tolerance, unity, and peaceful coexistence across the state and the country at large.
He described the coincidence in the commencement dates as a reminder of shared values between Islam and Christianity.
“The simultaneous commencement of Ramadan and Lent is a divine reminder that we all worship the same Almighty God. It is a call for unity, love, and mutual understanding among us,” he stated.
Ajadi urged Muslims and Christians to use the sacred periods of fasting and spiritual purification to pray fervently for Nigeria, especially in view of the nation’s economic and security challenges.
“Our country is facing significant hardship. The economic difficulties and prevailing insecurity require sincere prayers. This season of spiritual purification offers us a unique opportunity to seek God’s intervention for our nation,” he said.
He further emphasized that both Islam and Christianity preach peace, tolerance, and respect for constituted authority, urging citizens to embody these teachings in their daily lives.
“Let us live peacefully, tolerate one another, and continue to pray for those in leadership. Our faiths teach us to respect and uphold our leaders in prayer,” Ajadi added.
The PDP chieftain concluded by wishing Muslims a spiritually fulfilling Ramadan and Christians a reflective and enriching Lenten season, encouraging both communities to embrace love, sacrifice, and harmonious living throughout the sacred periods.
-

 celebrity radar - gossips6 months ago
celebrity radar - gossips6 months agoWhy Babangida’s Hilltop Home Became Nigeria’s Political “Mecca”
-

 society6 months ago
society6 months agoPower is a Loan, Not a Possession: The Sacred Duty of Planting People
-

 society5 months ago
society5 months agoReligion: Africa’s Oldest Weapon of Enslavement and the Forgotten Truth
-

 news6 months ago
news6 months agoTHE APPOINTMENT OF WASIU AYINDE BY THE FEDERAL GOVERNMENT AS AN AMBASSADOR SOUNDS EMBARRASSING








