Editorial
WHO confirms more Ebola cases in Congo
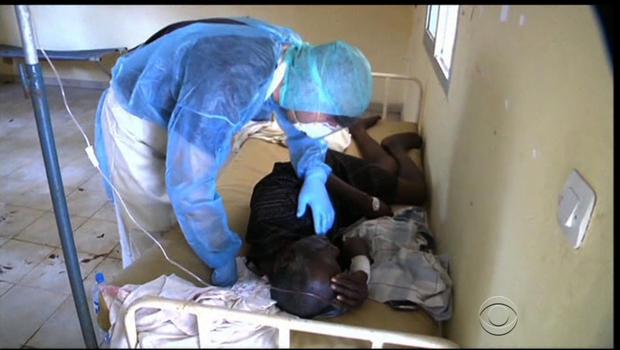
The World Health Organisation on Monday confirmed that a second Ebola case in Congo.
“So far there are 19 suspect cases, including three deaths and two lab-confirmed cases,” a WHO spokesperson in Geneva said via e-mail.
The first case was confirmed on Friday in Bas-Uele province in the north-east.
The WHO has said the outbreak appears to be limited to that remote area, and that there is no need for travel restrictions for the time being.
The Africa Centres for Disease Control and Prevention, a continent-wide mechanism to monitor disease outbreaks, said it had activated its emergency operational centre to monitor the situation in Congo.
The Central African country has suffered seven previous outbreaks of Ebola since the virus was discovered in the country in 1976.
The last outbreak, in 2014, left 49 people dead.
The haemorrhagic fever has been most detrimental in West Africa, where it claimed more than 11,000 lives in 2014 to 2015.
The WHO declared Guinea, Liberia and Sierra Leone, the three countries that had been most effected by the epidemic, free of Ebola in 2016.
NAN reports that the GAVI global vaccine alliance said on Friday some 300,000 emergency doses of an Ebola vaccine developed by Merck could be available in case of a large-scale outbreak, after the WHO confirmed a fatal case in Congo.
The vaccine, known as “rVSV-ZEBOV”, was shown to be highly protective against Ebola in clinical trials published in December 2016.
NAN reports that the experimental Ebola vaccine was highly protective against the deadly virus in a major trial in Guinea, according to results published in The Lancet.
The vaccine is the first to prevent infection from one of the most lethal known pathogens, and the findings add weight to early trial results published in 2016
The vaccine was studied in a trial involving 11, 841 people in Guinea during 2015.
Among the 5,837 people who received the vaccine, no Ebola cases were recorded 10 days or more after vaccination.
In comparison, there were 23 cases 10 days or more after vaccination among those who did not receive the vaccine.
The trial was led by WHO, together with Guinea’s Ministry of Health, Medecins sans Frontieres and the Norwegian Institute of Public Health, in collaboration with other international partners.
“While these compelling results come too late for those who lost their lives during West Africa’s Ebola epidemic, they show that when the next Ebola outbreak hits, we will not be defenceless,” said Marie-Paule Kieny, WHO’s Assistant Director-General for Health Systems and Innovation, and the study’s lead author.
The vaccine’s manufacturer, Merck, Sharpe & Dohme, this year received Breakthrough Therapy Designation from the United States Food and Drug Administration and PRIME status from the European Medicines Agency, enabling faster regulatory review of the vaccine once it is submitted.
Since Ebola virus was first identified in 1976, sporadic outbreaks have been reported in Africa.
The 2013–2016 West African Ebola outbreak, which resulted in more than 11 300 deaths, highlighted the need for a vaccine.
The trial took place in the coastal region of Basse-Guinée, the area of Guinea still experiencing new Ebola cases when the trial started in 2015.
The trial used an innovative design, a so-called “ring vaccination” approach, the same method used to eradicate small pox.
When a new Ebola case was diagnosed, the research team traced all people who may have been in contact with that case within the previous three weeks, such as people who lived in the same household, were visited by the patient, or were in close contact with the patient, their clothes or linen, as well as certain “contacts of contacts”.
A total of 117 clusters (or “rings”) were identified, each made up of an average of 80 people. (dpa/NAN)
Editorial
NGIJ Commences Governance Assessment Tour In Ondo State
The Nigerian Guild of Investigative Journalists (NGIJ) will on Monday commence Governance Assessment Tour in Ondo State to assess impact of Governor Oluwarotimi Akeredolu administration on the people.
A statement issued on Saturday by the Guild Public Relations Officer, Adeyemi Obadimu said NGIJ delegation would arrive Akure, the Ondo State Capital on Monday to commence the Governance Assessment.
Obadimu noted that the Governance Assessment aimed at rooting for the truth, getting firsthand information from different stakeholders and assessing impact of current administration in Ondo State.
”Our members from every part of the country will on Monday converge in Akure, Ondo State Capital for commencement of Governance Assessment Tour to assess Governor Oluwarotimi Akeredolu administration impact on the people.
”Our crew will engage critical stakeholders including traditional rulers, civil servants, labour unions, pensioners, top government officials, students, market men/women and others in order to get firsthand information about the current administration led by Governor Akeredolu.”
Obadimu disclosed that the guild has carried our similar assessment in Kogi and Bayelsa states, saying ‘this is part of our contributions to deepen Democratic rule in Nigeria’.
Business
Heritage bank partners with Multi choice to flag off BBN third edition

Sequel to successful outing of season-2 of the Big Brother Naija (BBNaija) last year, Heritage Bank Plc, has again partnered with MultiChoice Nigeria Limited, owners of the DSTV and GOTV brands to bring the third season of the entertainment to viewers across Africa and beyond.
The Big Brother Naija 2018 was launched on Sunday night with Ebuka Obi-Uchendu as the host with 20 house mates battling for the winning prize of N45 million which include a cash gift of N25 million and SUV Jeep among others. The theme of this year’s edition is “Double Wahala.”
The host said one of the innovations in this season was that viewers could give housemates secret tasks to do and some lucky viewers could win cash prizes of N1 million during the duration of the show.
The house mates will stay at the Big Brother House for the next 85 days to entertain viewers and the show promises to show case a lot of drama, intrigues and romance.
Speaking at the live streaming of the opening session in Lagos at the weekend, Mr. Fela Ibidapo, Group Head, Corporate Communications of Heritage Bank said, “When the last season of the reality show was about to start, some of us did know what we were getting into. We are back here again because last year was successful.’’
He said Heritage Bank believed in creativity and innovation and expressed appreciation to MultiChoice for the opportunity to be part of this edition of the show.
About 20 house mates were invited to the house in the third season of the show which began last Sunday night, with four of them debuting in a reality show for the first time in their lives.
This is an increase over 12 that participated in last year’s edition.
The new comers are Nina Chinoso 21 years- old, Vanessa Williams aka Vandora from Edo State, Teddy A 29 and Kelvin Burle aka K-Brule 23 years-old.
The others are Alexandria (a lady) 22, Adedayo Adewunmi aka Dee-One, a comedian; Princess Onyejekwe 25, Miracle Igbekwe, a pilot and model; Ahneeka 25 years and a TV presenter; Rico Savey,25; Bam Bam,28 years-old freelancer in acting and singing; Bitto Brain 26, Ifu Ennanda, an actress and business broker; Leo 25 years-old corporate hustler with a retail firm; Khloe 24 and a fashion designer; Angela 31 years-old film maker, Anto, Tobi- 23, Cee-C and Omololu Adetokunbo aka Lolu.
In the season two of the show, besides providing financial assistance to the BB Naija contenders, Heritage Bank helped them with business and advisory support services for between six to nine months.
This support helped to chart a veritable course and equip the housemates with the necessary tools to make informed business choices during their individuals’ careers.
In a bid to bring additional value to Nigerian culture, Heritage Bank also ensures that in partnering with the housemates, they take positive steps towards creating, preserving and transferring wealth to Nigerians.
Heritage Bank had also organised an SME enhancement capacity programme for the Ex-Housemates of the BB Naija and other emerging 21st century entrepreneurs at an SME enhancement capacity training programme.
Big Brother Nigeria was designed by its creators to attract controversies and create agenda for social discussions.
Business
How civil defence corps became a millionaire overnight with Skye bank Promo


Mrs Eunice Odubor, a 48- year old staff of the Civil Defence Corps, yesterday won the 4th edition of the season 2 reach for the Skye millionaire reward.
Forty other lucky loyal customers across Nigeria also went home with various cash gifts ranging between N20, 000, N50, 000 and N100, 000 respectively.
The cash bonanza and draw which took place at the busy Ekiosa market in Benin City, the Edo State Capital, had in attendance top management staff and Zonal officers of National lottery Commission which witnessed the occasion.
Presenting the cheque to Mrs Isibor, at the resident of the Obazelu of Benin Kingdom, Chief Osaro Idah, the group head Product and Innovation of the bank, Ndubisi Osakwe, said the presentation was a clear demonstration of Skye Bank’s commitment to continue to impact the lives of loyal customers.
He said: “Today Skye Bank has shown its capacity in supporting individuals and retail businesses. We started this journey a couple of months back…now in its 3rd year and this is the fourth in the series.
“We have given given rewards to customers who are loyal to Skye Bank, who have responded to our promo to put N10, 000 for 30 days in savings account and they will qualify to win up to N1m. Mrs Idubor of Foresty branch Benin who has been loyal invested N10, 000 and has won N1m and her account will be credited”
In his remark, Chief Osaro Idah, who presented the cheque to the winner commended the bank for blazing the trail in corporate social responsibility among banks.
Elated Mrs Idubor, who could not hold back tears expressed her gratitude to Skye Bank for the money and promised to tell others to bank with Skye Bank.
She said part of the amount will be invested into the education of her child who had dropped out of the Ambrose Alli University Ekpoma due to paucity of funds.
The Zonal Coordinator, National Lottery Commission, Mrs Kate Okocha, also commended the bank for the transparency and encouraging people to save by opening account.
-

 celebrity radar - gossips6 months ago
celebrity radar - gossips6 months agoWhy Babangida’s Hilltop Home Became Nigeria’s Political “Mecca”
-

 society6 months ago
society6 months agoPower is a Loan, Not a Possession: The Sacred Duty of Planting People
-

 society5 months ago
society5 months agoReligion: Africa’s Oldest Weapon of Enslavement and the Forgotten Truth
-

 news6 months ago
news6 months agoTHE APPOINTMENT OF WASIU AYINDE BY THE FEDERAL GOVERNMENT AS AN AMBASSADOR SOUNDS EMBARRASSING










You must be logged in to post a comment Login